
Separating Used Oil Using Filtration & Ceramic Zeolite


Fuel oil is an important energy source derived from petroleum and is used as fuel in various fields of industry, as well as transportation. the depletion of petroleum reserves, also cannot be renewed, Currently, many people are competing to find alternative energy from petroleum that can be renewed so that its availability can be guaranteed.
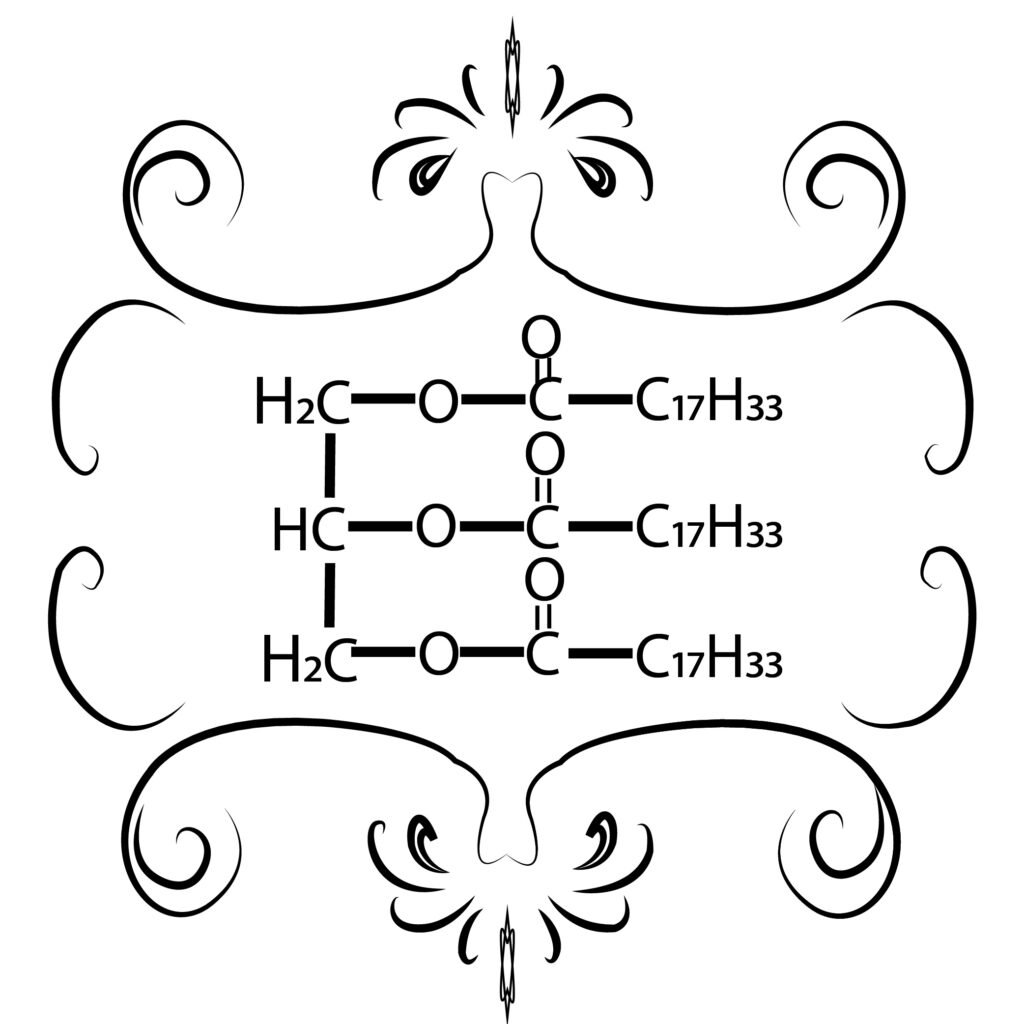
Biodiesel is a chemical process in the form of methyl and ester compounds from the esterification and transesterification process of vegetable oils or animal fats or one of the alternative fuels produced from natural and renewable materials, such as palm oil, castor oil, soybean oil, while examples of animal fats include beef fat, lard, and other animal fats. Biodiesel is usually made from the esterification/transesterification process of vegetable oils or animal fats using alcohol, Zeolites here have a role as a catalyst or help speed up chemical reactions. The use of biodiesel has several advantages compared to using diesel oil as follows:
1.) The use of biodiesel is more environmentally friendly when compared to fuel from petroleum because it produces better emissions.
2.) has a higher cetane number (>57) so that the combustion process is better
3.) has lubricating properties against engine pistons
4.) biodiesel can be renewed or produced continuously because the raw material for biodiesel uses renewable vegetable oils or animal fats. 5.) increase to supply of own fuel because it can be produced locally.

Materials that can be used for the manufacture of biodiesel can use compounds contained in pork fat or others in the form of triglycerides, triglycerides when reacted with alcohol will produce esters and glycerol. This process is often called a transesterification reaction. While the esterification reaction is a nucleophilic ion substitution reaction with an acid catalyst. Homogeneous catalysts are catalysts that are soluble in the reactants or reaction products, while heterogeneous catalysts are catalysts that are different from the reactants or products. Heterogeneous catalysts have several advantages compared to homogeneous catalysts, including having high catalytic activity, being more stable during transesterification or esterification, and being is easily separated.

Zeolite is a heterogeneous catalyst and contains cations K+ (Potassium), Na+ (Sodium), Ca2+ (calcium), and Mg2+ (Magnesium) while synthetic zeolite usually only contains K+ (calcium), and Na+ (Sodium). Zeolite has a hollow structure or pores, usually, this cavity is widely used as an adsorbent, filter, natural cation exchanger, and used as a catalyst. The advantages of using clinoptilolite natural zeolite as a catalyst in the manufacture of biodiesel :
1.) clinoptilolite natural zeolite has a solid form and cavity, so zeolite can be very suitable to be used as a catalyst.
2.) able to increase ester purity.
3.) Easier to separate than a liquid catalyst.
the use of zeolites can also streamline the process of acid esterification and base transesterification where the process is carried out using zeolites can be done simultaneously or not separately to save time, energy, and others.
From the explanation above, it can be concluded that the use of zeolite as a catalyst is very helpful in making very practical biodiesel.
Zeolite (Zeinlithos) or also referred to as boiling, in chemist’s research has long been the center of attention. Every year, various research journals around the world, always contain zeolites for various applications, especially those directed at the aspects of improving and efficiency of industrial processes and environmental pollution.
Generally defined as a three-dimensional structure of silica-alumina crystals, which are formed from tetrahedron of alumina and silica with internal cavities containing metal ions, usually alkaline or alkaline earth, and free moving water molecules. Empirically, the molecular formula for zeolite is Mx / n. (AlO2) x. (SiO2) y.xH2O. So far, the zeolite structure is known to vary, but in general, the structure is formed from the primary structural unit, in the form of a tetrahedral which then becomes the secondary polyhedral unit and forms a polyhedron and finally the zeolite structural unit.
In Indonesia, the number of zeolites is very abundant and scattered in various regions both on the islands of Java, Sumatra, and Sulawesi. The use of Indonesian zeolite for direct use cannot yet be carried out, because Indonesian zeolite contains a lot of impurities so that it needs to be processed first to remove or separate it from impurities.
Unique Properties of Zeolites
Because of the unique physical and chemical properties of zeolites, so in this decade, zeolites by researchers have become a versatile mineral. These unique properties include dehydration, adsorbent, and molecular filters, catalysts, and ion exchangers.
Zeolites have to dehydrate properties (releasing H20 molecules) when heated. In general, the zeolite frame structure will shrink. But the basic framework did not change significantly. Here the H2O molecule appears to have a specific position and can be reversibly removed. The nature of zeolites as adsorbents and molecular filters is possible because of the hollow structure of zeolites so that zeolites are able to absorb a large number of molecules that are smaller in size or according to the size of their cavity. In addition, zeolite crystals that have been dehydrated are selective adsorbents and have high adsorption effectiveness.

The ability of zeolites as catalysts is related to the availability of active centers in the channels between zeolites. These active centers are formed due to the presence of the Bronsted and Lewis type acid functional groups. The comparison of these two types of acids depends on the zeolite activation process and reaction conditions. These active acidic centers can then bind the base molecules chemically. While the nature of zeolite as an ion exchanger is due to the presence of alkali metal cations and alkaline earth. These cations can move freely in the cavity and can be exchanged with other metal cations with the same number. Due to the zeolite’s hollow structure, anions or molecules smaller or equal to the cavity can enter and get trapped.
Zeolite application
As mentioned above, in this decade, zeolite has been widely used by the public. Here are some examples of applications:
Neutralizing soil acidity, increasing soil aeration, supporting mineral sources in fertilizers and soil, and as an effective controller in the release of ammonium ions, nitrogen and potassium fertilizers.
Increasing the value of nitrogen efficiency, can reduce gastric disease in ruminants, control the humidity of animal waste, and the ammonia content of animal waste.
Cleaning fish pond water that has a water recirculation system can reduce nitrogen levels in the fish pond.
As a catalyst in the process of breaking down petroleum hydrocarbons, as panels for developing solar energy, and absorbing freon gas.
Fillers in the paper, cement, concrete, plywood, steel and cast iron industries, adsorbents in the textile and palm oil industries, raw materials for the manufacture of ceramics.
Thank you for reading our article. Keep in touch with Nusagri to get more information about zeolite. Nusagri.co.id is the pioneer in exploitation and processing of zeolite minerals in Indonesia used in Agricultural and multi sectors. Nusagri exported tons of zeolite for many countries including India and Malaysia. Please check our website and social media for further information about zeolite.
Water is a vital human life necessity. Directly water is needed for drinking, cooking, bathing, washing, and washing. Indirectly water is needed as part of the ecosystem with which life on earth can take place. However, water can also be a means of various toxic substances and pathogenic organisms that endanger humans. In developing countries today, nearly 25 million people die every year due to biological and chemical pollution in water. This is supported by the World Resource Institute report 1998-1999, that there are 1.4 million people worldwide who are not covered by safe drinking water supplies.
Groundwater often contains iron and manganese is quite high. In the water, these two metals are always together. For humans, both metals are essential but also toxic. Its presence in water can not only be detected in a laboratory but also can be recognized organoleptically. With a Fe or Mn concentration of at least 1 mg / L, the water feels bitter-acid, smells bad, and is brownish-yellow in color. On an industrial scale, Fe and Mn in water are usually reduced by aerating water at pH> 7 so that these two metals settle as oxides.
Another process is to bind Fe and Mn with a cation exchanger. Both of these methods cannot be carried out by the general public because they require expensive facilities, equipment, and materials, while conventional filtration using sand and fibers can only improve physical quality of water such as turbidity.
However, in fact in Indonesia available natural ion exchangers are cheap and easy to obtain. Zeolites are one of the most widely available natural ion exchangers. Zeolites are very abundant in the form of large pieces of rock that are exported. The ability of zeolites as ion exchangers has long been known and used as a chemical pollutant remover. In zeolite water also turns out to be able to bind E. coli bacteria.

This ability depends on the filtering rate and the ratio of the volume of water to zeolite mass. However, for metals the variables that influence the effectiveness of cation exchange are unknown. In order to obtain an easy, inexpensive, and reliable method and tool for treating groundwater containing high levels of Fe and Mn to be suitable as raw water for drinking water, a simple filtration system with natural zeolite as its cation exchanger was created. This experiment aims to determine the optimum contact time and filtration rate.
Physiologically, Fe and Mn double as essential metals but can also be toxic. The limit of the separator is the concentration. Fe is mainly present as a heme of hemoprotein, transferrin (transport protein), and ferritin (iron warehouse) molecules. Fe intakes that are too large can cause this metal to accumulate as ferritin. This compound is very toxic because it is in the form of Fe (OH) 3, the source of iron for lipid peroxidation reactions that can produce radicals that can ultimately interfere with cellular level oxidation and GSH. The ability of zeolites as iron-exchangers to produce reactive oxygen species has long been known, especially those related to cancer proliferation, which have been reported in various literature. This radical formation causes zeolites to reduce E. coli in water as found.
Based on the results of the study, Zeolite without treatment is effective enough to reduce the concentration of Fe and Mn in groundwater. The effectiveness of zeolite in reducing Mn concentration is better than Fe. The optimum filtering conditions for contact time are 30 minutes and for a filtration rate of 2 mL/minute. Filtering discharge with a 4 cm diameter glass column and a height of 50 cm, with a contact time of 30 minutes and a filtration rate of 2 mL/minute is still very small which is only enough for one person to drink per day.